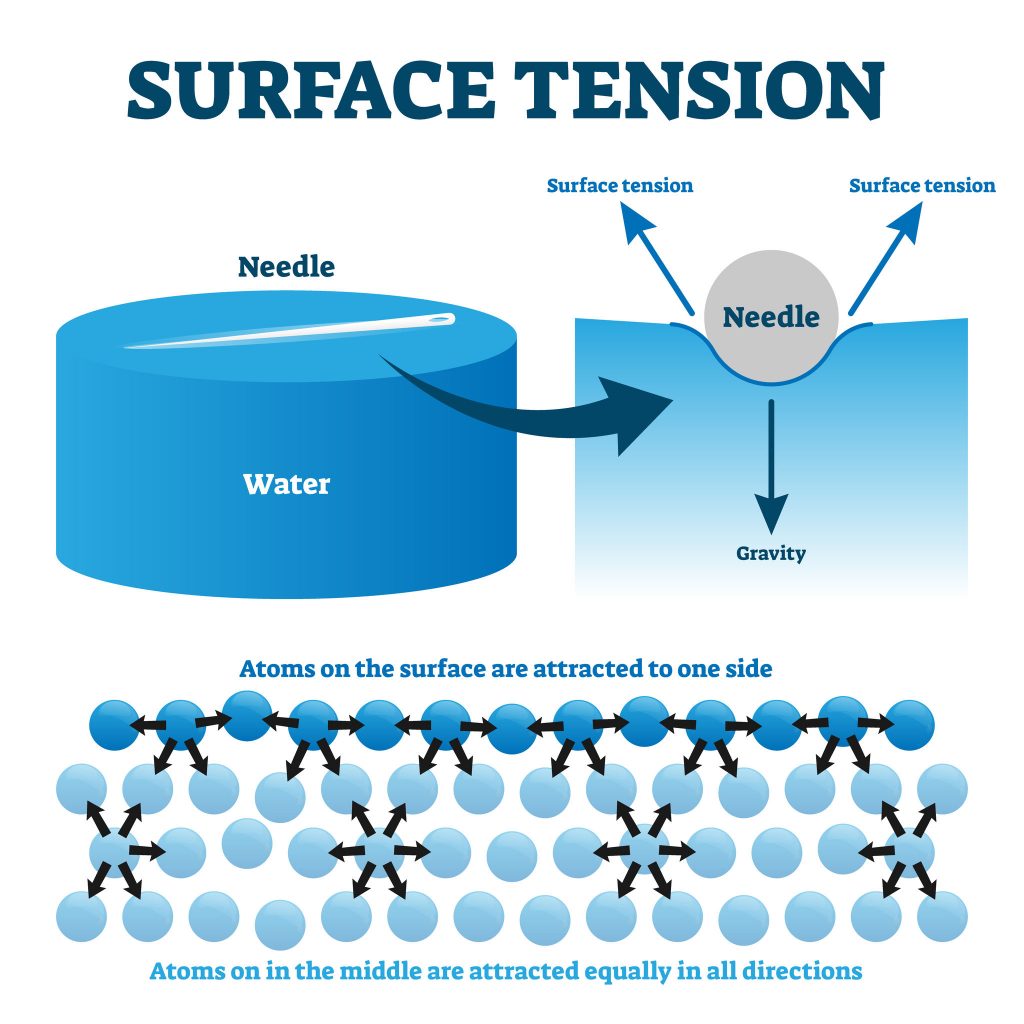
How Does The Surface Tension Of Water Compare To Other Liquids . They will compare the way water. the answer is surface tension—the way that a liquid surface acts a little like a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. on the surface, the molecules are attracted from the sides and bottom. No water molecules exist on the top; learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. Liquids that have strong intermolecular forces, like the hydrogen bonding in. Hence, no attractive forces from the top. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. the surface tension of a liquid is a measure of the elastic force within the liquid's surface.
from www.australianenvironmentaleducation.com.au
in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Hence, no attractive forces from the top. on the surface, the molecules are attracted from the sides and bottom. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. the answer is surface tension—the way that a liquid surface acts a little like a. No water molecules exist on the top; surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in. They will compare the way water.
Water is found everywhere on earth, so why is it important?
How Does The Surface Tension Of Water Compare To Other Liquids learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. on the surface, the molecules are attracted from the sides and bottom. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. the answer is surface tension—the way that a liquid surface acts a little like a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. No water molecules exist on the top; They will compare the way water. Hence, no attractive forces from the top. Liquids that have strong intermolecular forces, like the hydrogen bonding in.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does The Surface Tension Of Water Compare To Other Liquids in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. No water molecules exist on the top; surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. on the surface, the molecules are attracted from the sides and bottom. Liquids. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download How Does The Surface Tension Of Water Compare To Other Liquids No water molecules exist on the top; the surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen bonding in. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. in comparison,. How Does The Surface Tension Of Water Compare To Other Liquids.
From errantscience.com
Water surface tension ErrantScience How Does The Surface Tension Of Water Compare To Other Liquids Hence, no attractive forces from the top. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. on the surface, the molecules are attracted from the sides and bottom. Liquids. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.youtube.com
DETERMINING THE SURFACE TENSION OF WATER EASY TO UNDERSTAND YouTube How Does The Surface Tension Of Water Compare To Other Liquids No water molecules exist on the top; Liquids that have strong intermolecular forces, like the hydrogen bonding in. the answer is surface tension—the way that a liquid surface acts a little like a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of. How Does The Surface Tension Of Water Compare To Other Liquids.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts How Does The Surface Tension Of Water Compare To Other Liquids on the surface, the molecules are attracted from the sides and bottom. No water molecules exist on the top; the answer is surface tension—the way that a liquid surface acts a little like a. They will compare the way water. Liquids that have strong intermolecular forces, like the hydrogen bonding in. learn how water molecules cohere to. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water How Does The Surface Tension Of Water Compare To Other Liquids Hence, no attractive forces from the top. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. on the surface, the molecules are attracted from the sides. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does The Surface Tension Of Water Compare To Other Liquids on the surface, the molecules are attracted from the sides and bottom. Hence, no attractive forces from the top. Liquids that have strong intermolecular forces, like the hydrogen bonding in. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. in comparison, organic liquids, such as benzene and alcohols, have. How Does The Surface Tension Of Water Compare To Other Liquids.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does The Surface Tension Of Water Compare To Other Liquids No water molecules exist on the top; Liquids that have strong intermolecular forces, like the hydrogen bonding in. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. the answer is surface tension—the way that a liquid surface acts a little like a. They will. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Does The Surface Tension Of Water Compare To Other Liquids Liquids that have strong intermolecular forces, like the hydrogen bonding in. on the surface, the molecules are attracted from the sides and bottom. They will compare the way water. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. the answer is surface tension—the. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.researchgate.net
Comparison of water and liquid metal (LM). a) The surface tension of How Does The Surface Tension Of Water Compare To Other Liquids the surface tension of a liquid is a measure of the elastic force within the liquid's surface. the answer is surface tension—the way that a liquid surface acts a little like a. Hence, no attractive forces from the top. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. . How Does The Surface Tension Of Water Compare To Other Liquids.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does The Surface Tension Of Water Compare To Other Liquids Hence, no attractive forces from the top. the answer is surface tension—the way that a liquid surface acts a little like a. Liquids that have strong intermolecular forces, like the hydrogen bonding in. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension is the energy, or work,. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does The Surface Tension Of Water Compare To Other Liquids in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. the answer is surface tension—the way that a liquid surface acts a little like a. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. No water. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download How Does The Surface Tension Of Water Compare To Other Liquids Liquids that have strong intermolecular forces, like the hydrogen bonding in. the answer is surface tension—the way that a liquid surface acts a little like a. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. No water molecules exist on the top; surface. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does The Surface Tension Of Water Compare To Other Liquids No water molecules exist on the top; learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. on the surface, the molecules are attracted from the sides and bottom. surface tension is the energy, or work, required to increase the surface area of a. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID How Does The Surface Tension Of Water Compare To Other Liquids No water molecules exist on the top; the surface tension of a liquid is a measure of the elastic force within the liquid's surface. They will compare the way water. Liquids that have strong intermolecular forces, like the hydrogen bonding in. surface tension is the energy, or work, required to increase the surface area of a liquid due. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.youtube.com
Surface Tension of given Liquid (Water) by Jaeger's Method YouTube How Does The Surface Tension Of Water Compare To Other Liquids on the surface, the molecules are attracted from the sides and bottom. Hence, no attractive forces from the top. the answer is surface tension—the way that a liquid surface acts a little like a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. They will compare. How Does The Surface Tension Of Water Compare To Other Liquids.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint How Does The Surface Tension Of Water Compare To Other Liquids Liquids that have strong intermolecular forces, like the hydrogen bonding in. learn how water molecules cohere to form a strong surface layer that resists external forces and allows some objects to float on it. the answer is surface tension—the way that a liquid surface acts a little like a. They will compare the way water. surface tension. How Does The Surface Tension Of Water Compare To Other Liquids.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter How Does The Surface Tension Of Water Compare To Other Liquids surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Hence, no attractive forces from the top. No water molecules exist on the top; Liquids that have strong intermolecular forces, like the hydrogen bonding in. They will compare the way water. on the surface, the molecules are attracted. How Does The Surface Tension Of Water Compare To Other Liquids.